Corgi Health Testing Information
The health of our dogs and puppies is our top priority.
We genetically test all of our breeding corgis to ensure that we are producing the best quality corgi puppies we possibly can with no known inherited diseases.
** Health Certificates are available on request for all our dogs. **
(All health testing descriptions are from the GenSol Diagnostics)
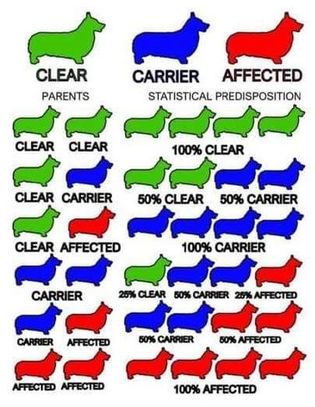
Carrier Mating Chart
DEGENERATIVE MYELOPATHY (DM)
VON WILLEBRAND'S DISEASE TYPE I (VWD1)
EXERCISE INDUCED COLLAPSE (EIC)
Degenerative Myelopathy (DM) is a progressive disease of the spinal cord in older Corgi dogs. The disease has an insidious onset typically between 8 and 14 years of age. It begins with a loss of coordination (ataxia) in the hind limbs. The affected dog will wobble when walking, knuckle over or drag the feet. This can first occur in one hind limb and then affect the other. As the disease progresses, the limbs become weak and the dog begins to buckle and has difficulty standing. The weakness gets progressively worse until the dog is unable to walk. The clinical course can range from 6 months to 1 year before dogs become paraplegic. If signs progress for a longer period of time, loss of urinary and fecal continence may occur and eventually weakness will develop in the front limbs. Another key feature of DM is that it is not a painful disease. Although any dog can be tested for DM, it is possible that the genetic background that predominates in some breeds prevents the development of symptoms even in dogs testing affected (at risk). At this time the required evidence of association between the genetic mutation and actual spinal cord evaluations has only been proven in the breeds listed.
Please see http://www.ofa.org/dnatesting/dmexplanation.html and http://www.caninegeneticdiseases.net/DM/ancmntDM.htm for additional information on DM diagnosis.
EXERCISE INDUCED COLLAPSE (EIC)
VON WILLEBRAND'S DISEASE TYPE I (VWD1)
EXERCISE INDUCED COLLAPSE (EIC)
Exercise Induced Collapse (EIC) is a canine genetic disorder that leads to loss of muscle control following periods of extreme exercise. Episodes generally occur after 5-25 minutes of excessive activity that can include actively running for extended periods of time. Episode severity ranges between different dogs and often begins with a form of rocking followed by weakening of the hind limbs and eventual collapse. Attacks are typically brief (less than 20 minutes) and dogs tend to recover. In a limited number of cases, episodes can be fatal. Affected dogs begin to show symptoms from a couple of months to 3 years of age and are more susceptible at an age when more intensive training begins. It is important for owners of dogs affected with EIC to be familiar with activities that may trigger an episode.
VON WILLEBRAND'S DISEASE TYPE I (VWD1)
VON WILLEBRAND'S DISEASE TYPE I (VWD1)
VON WILLEBRAND'S DISEASE TYPE I (VWD1)
Von Willebrand disease (vWD) is a genetic disorder that prevents normal blood clotting and can cause extended bleeding following injury. The disorder results from a deficiency or lack of sufficient von Willebrand factor (vWf) which functions as a binding protein during blood clotting. Three types of vWD have been identified in dogs to date and are known as vWD type 1, 2 and 3. Within these three types there are five different genetic mutations that are currently known that lead to canine vWD. Von Willebrand’s disease type 1 (VWD1) results in reduction in normal levels of vWf to approximately 5-10% of normal. Since some vWf is produced in dogs homozygous for the VWD1 mutation, this form of the disorder is considered to be less serious than type 2 and 3. The mutation (G>A substitution) has variable penetrance and is recessive requiring two copies of the mutation in affected dogs. Typical symptoms of the disease encompass excessive or abnormal bleeding following injury or the presence of blood in various bodily secretions (urine, feces, etc.).
HEREDITARY CATARACTS (HC)
PROGRESSIVE RETINAL ATROPHY ROD-CONE DYSPLASIA 3 (PRA-RCD3)
VON WILLEBRAND'S DISEASE TYPE I (VWD1)
Cataracts are a clouding of lens of the eye caused by a breakdown of tissue in the eye. This generally results in an inability to see clearly, and can cause total blindness. In canines, mutations that result in cataracts can be passed to offspring and is known as Hereditary Cataracts (HC), Juvenile Cataracts (JC) or Early Onset cataracts (EOC). A mutation in the HSF4 gene causes this type of cataracts in several breeds of dogs. In this case, the dog is typically affected bilaterally with both eyes affected by the disease. The cataracts associated with HSF4 also occur in the posterior region of the lens. They usually begin small and grow progressively, though the speed of growth is highly variable. Some cataracts will grow so slowly that the dog’s vision remains relatively clear, while others will grow such that the dog will quickly go blind. Corrective surgery is possible, though it is costly and is not always effective. One HSF4 mutation causes the recessive form of HC in Boston Terriers, Staffordshire Bull Terriers, and French Bulldogs. Because it is recessive, a dog must have two copies of this mutation to experience this form of cataracts. This mutation is only responsible for early-onset HC, which typically occur between 12 months and 3 years of age in Staffordshires, and between 2-3 years in Boston Terriers. Boston Terriers can also be afflicted by late-onset HC; however, the HSF4 gene mutation is not responsible for that particular form of cataracts. A separate mutation of the HSF4 gene is responsible for HC in Australian Shepherds. This mutation affects Aussies differently, in that the disease is dominant, but not completely penetrant. This means that only one copy of the mutation is necessary to predispose a dog to the disease, however, incomplete penetrance means that a dog that has this mutation will not always develop HC. Research suggests that the mutation makes a dog 12 times more likely to develop posterior bilateral cataracts at some point in their lifetime. It is likely that a secondary gene interaction occurs in the small percentage of dogs possessing the HC mutation but do not develop cataracts, however, this interaction is not yet know. It is important to note that not all cataracts are hereditary. Cataracts can also be caused by old age or injury. Also, cataracts that occur in different regions of the lens can also be familial, but not necessarily attributed to this gene mutation.
PROGRESSIVE RETINAL ATROPHY ROD-CONE DYSPLASIA 3 (PRA-RCD3)
PROGRESSIVE RETINAL ATROPHY ROD-CONE DYSPLASIA 3 (PRA-RCD3)
PROGRESSIVE RETINAL ATROPHY ROD-CONE DYSPLASIA 3 (PRA-RCD3)
Progressive retinal atrophy (PRA) is a category of different progressive conditions leading to retinal atrophy and potential blindness. Cardigan Welsh Corgi, Pembroke Welsh Corgi, Chinese Crested and Pomeranian breeds can be affected by a particular type of PRA known as PRA-RCD3. Disease symptoms can present as early as 1 year old or earlier and are typically observed during an eye exam. The rod cells of the eye which are responsible for vision in low-light (night time) show gradual deterioration and the dog rapidly exhibits “night blindness” which is typically seen as a gradual inability for the dog to go outside at night due to loss of night vision. At approximately 2-3 years of age, the dog’s cone cells begin to degenerate. The disease continues to progress leading to a loss of color vision and vision in bright light and eventually results in complete blindness.
This website uses cookies.
We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.